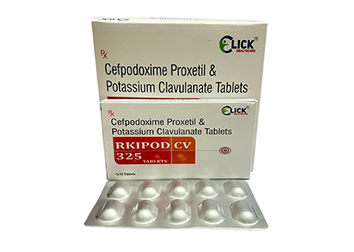
RKIPOD CV 325: Dual-Action Antibiotic Power
Click Healthcare introduces RKIPOD CV 325 (Cefpodoxime Proxetil and Clavulanic Acid Tablets), a powerful, synergistic antibiotic combination designed to tackle a broad range of bacterial infections, especially those resistant to standard treatments. This allopathic formulation is essential for treating conditions like respiratory tract infections (pneumonia, bronchitis), skin and soft tissue infections, urinary tract infections (UTIs), and otitis media. The dual-action approach ensures maximal effectiveness against both common and resistant bacterial strains, making it a critical asset in infectious disease management.
The efficacy of RKIPOD CV 325 stems from its two active components. Cefpodoxime Proxetil is a third-generation cephalosporin antibiotic that inhibits bacterial cell wall synthesis, leading to bacterial death. The addition of Clavulanic Acid is crucial; it acts as a beta-lactamase inhibitor, preventing resistant bacteria from deactivating Cefpodoxime. This protective shield allows the Cefpodoxime to effectively combat bacteria that produce the beta-lactamase enzyme, thus expanding the antibiotic's spectrum and potency against resistant organisms.
As a high-quality product under the Click Healthcare PCD Pharma Franchise Division of MK Healthcare, RKIPOD CV 325 is manufactured to the highest standards of safety and efficacy. This combination ensures that healthcare providers have a reliable tool for empirical treatment when resistance is suspected or confirmed. Our commitment to stringent quality control guarantees that this medication is a trustworthy option for patients requiring robust and comprehensive antibiotic therapy.
For pharmaceutical partners and professionals, RKIPOD CV 325 represents a strong opportunity within the Click Healthcare PCD Pharma portfolio. Given the rising challenge of antibiotic resistance, a highly effective combination product like this is always in demand. We welcome inquiries for partnership, franchise opportunities, or third-party manufacturing for this and other essential pharmaceutical formulations, contributing together to better patient outcomes in infection control.